ObamaCare Implant: ObamaCare Microchip RFID Myth







What is the Truth Behind the ObamaCare Microchip Implant Rumor?
We look at the truth behind the ObamaCare RFID chip myth that claims the Affordable Care Act contains mandatory microchip implants and data collection.
The ObamaCare RFID Microchip implant rumor, usually spread by email, is a claim that wording from the Affordable Care Act (the official name for ObamaCare) contains a section that requires the implantation of a RFID chip in all Americans by a certain date and allows for data collection from those devices.
The RFID implant rumor is one of the more pervasive ObamaCare myths. Learn about other ObamaCare myths here.
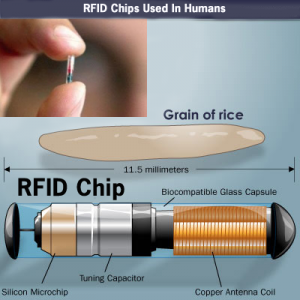
The photo above is an example of a RFID chip from a 2007 article discussing a glucose monitoring chip, although it is often attached to the RFID myth email, it has nothing to do with it.
Where Did the RFID Implant Rumor Originate?
The RFID implant rumor rises from a possibly intentional misreading of an old version of the Affordable Care Act, America’s Affordable Health Choices Act of 2009 HR 3200.
HR3200 was an unsuccessful bill introduced in the U.S. House of Representatives on July 14, 2009. Page 1001 of HR3200 included an amendment to section 519 of the Food, Drug, and Cosmetic Act, which allowed for data collection to “facilitate analysis of postmarket safety and outcomes data” from class II devices. A RFID chip is classified as a class II device by the FDA). None of the wording about class II devices appears in the Patient Protection and Affordable Care Act HR 3590 (Obamacare), although section 519 of the Food, Drug, and Cosmetic Act still includes an unamended passage on rules for class II devices and data collection from them.
If you want to see for yourself that the RFID chip rumor is a myth, you can do a “search and find.” Go to the official certified full-text versions of the Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act. You will see that there is nothing in the law about a RFID implant or data collection from a class II device.
The Section of the Law in Question
As mentioned above, America’s Affordable Health Choices Act of 2009 HR 3200 (an old version of ObamaCare that did not pass), contains an amendment to the Food, Drug, and Cosmetic Act, which most likely sparked the ObamaCare RFID implant rumor.
The excerpt below is from section 519 of the Food, Drug, and Cosmetic Act and was aimed at protecting the public from faulty medical devices. As you can see, there is no mandatory chip implant. However, it does allow data collection from implanted devices.
(A) is or has been used in or on a patient; and
(B) is—
(i) a class III device; or
(ii) a class II device that is implantable, life-supporting, or life-sustaining.(2) In developing the registry, the Secretary shall, in consultation with the Commissioner of Food and Drugs, the Administrator of the Centers for Medicare & Medicaid Services, the head of the Office of the National Coordinator for Health Information Technology, and the Secretary of Veterans Affairs, determine the best methods for—(A) including in the registry, in a manner consistent with subsection (f), appropriate information to identify each device described in paragraph (1) by type, model, and serial number or other unique identifier;(B) validating methods for analyzing patient safety and outcomes data from multiple sources and for linking such data with the information included in the registry as described in subparagraph (A), including, to the extent feasible, use of—
(i) data provided to the Secretary under other provisions of this chapter; and
(ii) information from public and private sources identified under paragraph (3);(C) integrating the activities described in this subsection with—
(i) activities under paragraph (3) of section 505(k) (relating to active postmarket risk identification);
(ii) activities under paragraph (4) of section 505(k) (relating to the advanced analysis of drug safety data); and
(iii) other postmarket device surveillance activities of the Secretary authorized by this chapter; and…3(B) In this paragraph, the term ‘data’ refers to information respecting a device described in paragraph (1), including claims data, patient survey data, standardized analytic files that allow for the pooling and analysis of data from disparate data environments, electronic health records, and any other data deemed appropriate by the Secretary.
To learn more about the uses of RFID chips in the healthcare field, please read the linked section of this wiki article on RFID and healthcare.
How Does the FDA Classify Devices Like RFID?
The FDA classifies different types of devices as Class I, II, or III. These “regulatory classes” are based on the level of control necessary to assure the safety and effectiveness of a given device.
Class I devices are things like dental instruments and tongue depressors. Class II devices are more complicated and include infusion pumps and surgical needles. Class III devices are complicated implantable medical devices like cardiac pacemakers and heart valves. These must be FDA approved and go through rigorous postmarket monitoring.
RFID chips used for patient identification and health information are considered class II devices. You can check out this document issued on December 10, 2004, that discusses guidance for RFID systems used for patient identification and health information. The same section of the law also contains mention of class III devices. The passage was intended to allow feedback about devices like a person’s pacemaker’s performance to improve health outcomes and the quality of the devices themselves.
Obamacare RFID Implant Myth Summary
To summarize, an early version of the law (HR3200) contained wording that potentially opened a gateway for collecting data from RFID implants. The language was dropped before the current law was passed.
The final version of the ACA includes sections that allow for different types of electronic data collection, albeit not from a class II device such as a RFID. It includes protocols for the transfer of medical records between physicians, guidelines on the collection of non-medical data for quality control, new standards for codification, and more.
A mandatory ObamaCare RFID implant is a myth, but RFID chips aren’t. RFID use may well be something that we, as a people, should consider discussing shortly. Although they have a lot of potential medical and safety benefits, they may also pose risks to privacy, some of which are illustrated in the video below:
ObamaCare RFID Facts
Here are a few facts about ObamaCare and RFID chips to help you understand the truth and the myths behind them.
• The only reference to “chip” in the ACA is CHIP (Children’s Health Insurance Program).
• There is no mention of mandatory RFID implants in the Affordable Care Act, but there was mention of data collection from class II devices in an early version of the law (HR3200).
• RFID chips are real, and there is more than a decade’s worth of legislation about their use.
• Georgia, California, North Dakota, Wisconsin, and Virginia have all passed legislation that prevents mandatory RFID implantation.
• RFID chips don’t have to be implanted. For instance, they can be put on credit cards or animal tracking collars.
• The FDA has shown some RFID implants to be safe for implantation in humans. A version of the RFID chip is currently in use as an animal tracker, either implanted or used in a detachable collar. These are used to track household pets and monitor endangered species. RFID chips are currently in use in ankle bracelets or wrist tags to track both vulnerable newborn babies and make sure they are only taken out of a hospital by their families, and to monitor of people in the correctional system, such as those released from facilities.
• While the ACA doesn’t allow for data collection from class II devices, it does contain wording about electronic data collection to improve the quality of care and better coordination between doctors including SEC. 3015. DATA COLLECTION; PUBLIC REPORTING.
• It is reasonable to think that, in the future, RFID chips will be in more widespread use. They could be implanted or be used in identity cards, alert bracelets and such. Not only could implanted chips monitor your health status if you were diabetic or prone to seizures, but they could be used as GPS, a means to transfer currency, cell phones, computers, etc.
• Like the use of drones and other new technology that could potentially be used to limit freedom rather than expand it, the implications of RFID devices need to be discussed openly by the public. The way we regulate RFID chips, and their usage will determine the dangers they pose to us.
• Despite the risks implants pose, there are also benefits. They are part of the new technology that we need to consider.
Examples of the ObamaCare RFID Chip Myth
The “chip myth” is usually spread through email, Facebook or some other form of social media communication. Please be aware that there are many forms of the myth, and they are all wrong. If you find others, please contact us and share it with us.
Here is one example of an early version of the rumor from back in 2009 before the bill was even signed into law.
Circulating since 2009 (various versions)
Status: False (see details below)Example:
Description: Rumor / Forwarded email
Circulating since 2009 (various versions)
Status: False (see details below)Example:
Email contributed by Sherry F., Feb. 11, 2013:
“Micro Chip Implant Coming March 23, 2013
The New Health Care (Obama care) law H.R. 3590 Also HR 4872 requires all US citizens to… have the RIFD implanted
This evil plan is being launched by America. It’s a microchip injected in your hand. It will contain all your personal data heath and bank accounts etc. It’s also a GPS device being monitored. They can deactivate it at any time if they find you suspicious or not loyal to their government or go against them or their system and you will lose everything you ever had. Soon this device will be made common just like they did credit cards. Turning paper money into digital money means nothing is physically in your hand. It will be made a must for every citizen with time according to their plan, and then they will spread it outside America so they can monitor and control as many people as they can and turn them into slaves with their digital technologies.
This device is the future or slavery
BEWARE of this EVIL DEVICE. If you don’t believe me do your own research before you come to argue or debate.
warn more people create this awareness do more research on your own and save yourself from this NEW DEVILRY.”
Here is an example of a version of the rumor from 2015 over 5 years after the law was passed.
“Rapture will take place any time from now. Everything hindering the rapture has been removed. Gospel has been preached almost everywhere, all the prophecies have been fulfilled. The devil is working very hard to occupy Christians with the things of this world so that the day will catch them unaware. Please be prepared, there is no more time. This is also a source of evangelism, souls are dying. God bless you!
Let’s Pray Hard: 6 6 6 The Mark Of The Beast Prophesy Finally Fulfilled… Written By: Jonathan Annobil. The US Senate has passed the Obama Health Bill into law. The implementation would commence soon. This bill would require all Americans to be implanted with a Radio Frequency Identification (RFID) chip in order to access medical care. The device will be implemented on the forehead or on the arm. This is to fulfill the prophesy in the Book of Revelation 13:15-18 concerning the MARK OF THE BEAST. Are you still doubting the END TIME? Do u know that the special car which was made for Obama is known as the BEAST? Get READY. The rapture is near! Revelations 13 is being played out right before us. Many are still unaware.1. Why is the chip being implanted exactly where the Bible says it would be. Why on the hand and forehead. Why not anywhere else?2. Why is it being connected to your bank account? Remember the Bible says you wont be able to buy or sell without the mark 6 6 6. And guess what! The chip is connected to your financial details. What breaks my heart the most is that many people in the Church will not make it if Jesus comes now? Many are unaware that the ends is near. Don’t tell me that its advancement in technology or development. If any area of your life is not in sync with God’s word repent and be converted. If you miss heaven you can never miss hell…think about it. Hell is not a pretty place, the worst part is that it is for eternity… He who has ears, let him hear what the Spirit says to the church. Please rather than post and forward senseless messages. Send to everyone you know. Do the work of an evangelist. PLEASE SHARE THIS MESSAGE WITH ALL YOUR CONTACTS.”
Other Variations of the ObamaCare Chip Myth
Other variations of the microchip myth include claims that ObamaCare will be implanting chips in babies, kids, or humans in general. Chip rumors also claim the chip will be implanted in the forehead, hand, or under the skin. RFID chip rumors also include specific dates for implantation such as 2016, 2017, or beyond. Other versions just remove the word ObamaCare, perhaps since the truth debunking these claims has become so widespread by now.
To reiterate, the chip myth is completely made up and spread through chain emails. The details change, but the facts remain the same. The PPACA is a law that first and foremost helps the sick and the poor. It uses technology in smart ways to advance health care but makes no mention of mandatory RFID chip implants or data collection from them.
Learning About RFID Chips
Did you see a different ObamaCare Micro Chip Implant Rumor? Check out our Myths Page for more information on Chip Implants. Check out the RFID videos below to learn more about manufacturers of implantable devices.
You can also learn more about RFIDs using the links below:
ObamaCare Microchip Implants and You: The Reality of RFID
Implanting microchips isn’t something ObamaCare does or can do. The Patient Protection and Affordable Care Act is a law that anyone can pick up and read. The law discusses data collection in general but makes no mention of mandatory implants or data collection from those implants.
As technology moves forward, we are sure to see a wide range of “smart devices” that can relay important information like health data and data about the device itself. It will be important for us to push aside the myths and focus on supporting smart legislation that allows us to use technology to improve health outcomes, without jeopardizing our safety or privacy.
ObamaCare Does Not Have a MicroChip Implant Mandate
![]()